Mechanism and harm of chromium fog
Mechanism and harm of chromium fog
According to archaeologists, the technology of electroless chromium plating has been used in bronze weapons in China 2200 years ago. The technology of electrolytic chromium plating was reported by gerther in his doctoral dissertation in 1856. In the 1920s, Sargent and Fink further developed the technology of hexavalent chromium plating using chromate as electrolyte, which led to the industrialization of chromium plating technology, So far, it has a history of nearly 100 years. Nowadays, many metal devices of household products and industrial products are coated with chromium. This is because the surface of chromium plating not only plays an aesthetic role, but also can improve the surface hardness and reduce the body metal corrosion. Although there are a variety of surface decoration and anti-corrosion technologies, chromium plating still occupies an important position. There are still a large number of chromium plating factories in the world, and the demand for chromium plating is strong.
According to different electrolytes, chromium plating process can be divided into hexavalent chromium process and trivalent chromium process. The hexavalent chromium Cr (VI) developed so far uses chromic anhydride (CrO3) as electrolyte and sulfuric acid as high concentration chromic acid solution (CrO3 + H2SO4). The acidity of the bath is very high. Chromium in chromic acid is reduced from Cr (VI) to Cr (III), and finally to Cr (0).
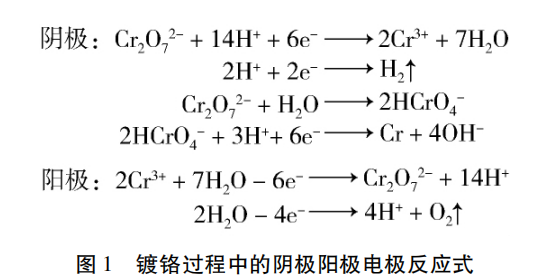
Fig. 1 reaction equation of cathode and anode in chromium plating process
In Cr (VI) electroplating process, due to the current efficiency of cathode is only 10% - 15%, and the insoluble lead alloy is used as anode, a large amount of hydrogen is generated at cathode and oxygen is generated at anode. When these gases rise to the liquid surface in the form of bubbles and release into the air, they carry a large number of chromium containing droplets and form fog like pollutants, as shown in Figure 2, commonly known as "chromium fog".
This kind of chromic acid discharged with chromium mist accounts for 20% - 40% of chromic acid used for chromium plating according to different working conditions. The production of chromium fog will not only cause a lot of loss of chromic anhydride, but also seriously affect the health of workshop workers due to the strong corrosiveness of chromic acid. Trivalent chromium uses chromium chloride (CrCl3) or chromium sulfate [CR2 (SO4) 3] as electrolyte. Although Cr (III) process has the characteristics of low concentration, wide range of current density and low toxicity, it is easy to produce pinholes and cracks due to its poor tolerance to impurities, and Cr (III) may be oxidized to Cr (VI) to pollute the electrolyte during the electroplating process, The trivalent chromium Cr (Ⅲ) process was applied in industry. At present, although trivalent chromium Cr (Ⅲ) process is developing rapidly, the mature hexavalent chromium Cr (Ⅵ) process is mainly selected for industrial chromium plating.
We can provide Fluorochrome fog inhibitor:
Potassium perfluorohexyl sulfonate (CAS No.3871-99-6)
perfluorohexyl ethyl sulfonic acid(CAS No.27619-97-2)
Contact us:
E-mail:info@brightchemical.com.cn
